Recommend that " Commercially available, PFS with medications that are already labeled should be used when possible.
To the greatest extent possible, provide adult IV push medications in a Ready To Administer form (to minimize the need for manipulation outside the pharmacy sterile compounding area) " (1)
Recommend that " Whenever possible, medications are dispensed in the most Ready To Administer form available from the manufacturer or, if feasible, in unit doses that have been repackaged by the pharmacy. " (3)
Recommend that " In adults, use IV push medications in an Ready To Administer form (to minimize the need for manipulation outside the pharmacy sterile compounding area).
Do not dilute or reconstitute IV push medications by drawing up the contents into a commercially available, prefilled flush syringe of 0.9% sodium chloride (USP).
Do not withdraw IV push medications from commercially available, cartridge-type syringes into another syringe for administration. " (3)
Recommend that " Whenever possible, medications should be available for inpatient use in unit-of-use and Ready-To-Administer packaging without further manipulation by the person administering the medication.
Every effort should be made to reduce situations where the person administering the medication has to withdraw doses from containers, reconstitute powdered drug products, split tablets, or perform other similar manipulations. " (2)
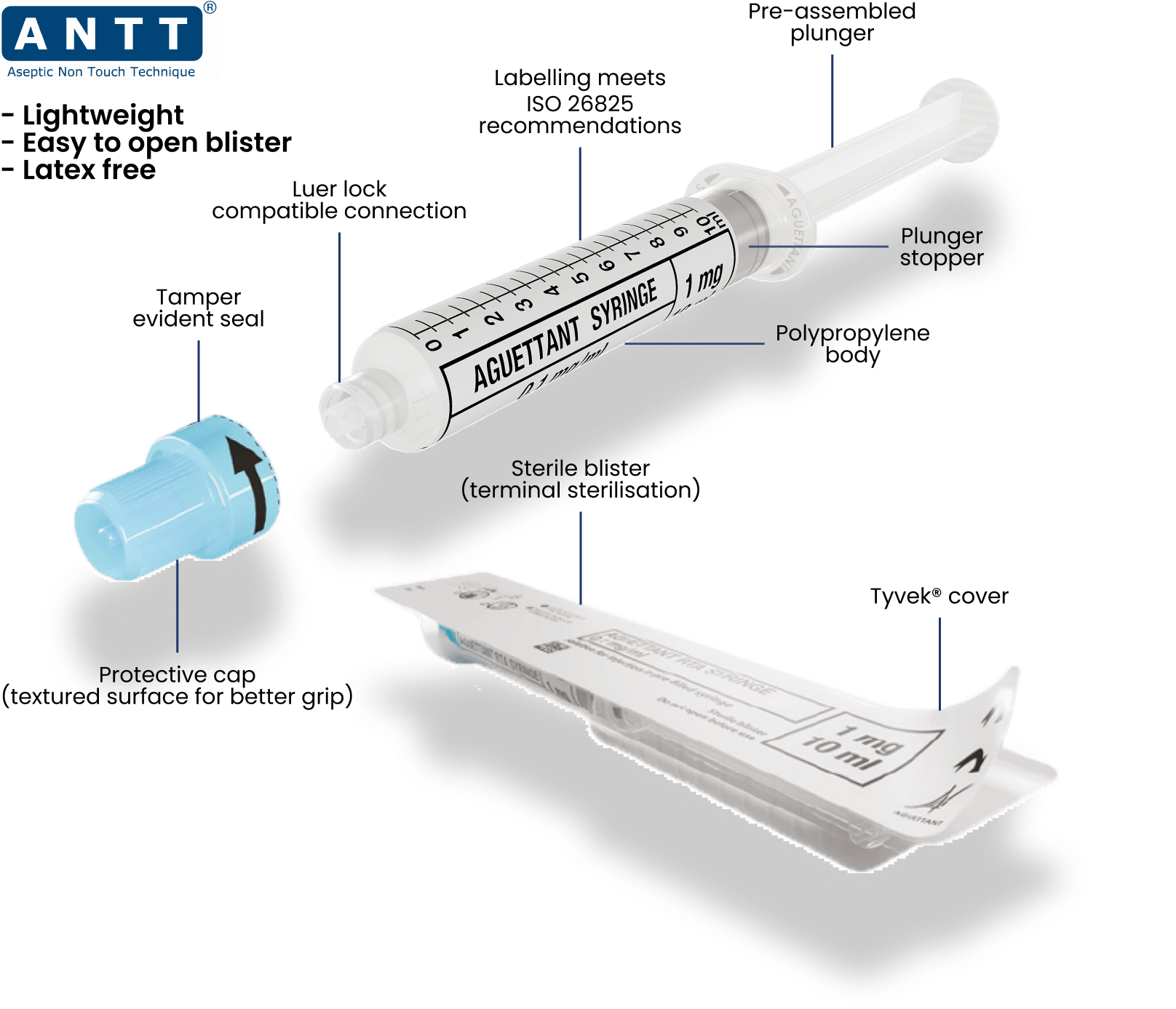
Our polypropylene syringes are pre-filled and ready-to-use in sterile packaging.
We strive to design innovative, ready-to-administer solutions to prevent medication errors, especially in emergency situations. (6)
When fast administration is essential, hand-preparing drugs at the bedside can result in delays in treatment. (6)
Ready-to-use pre-filled syringes eliminate preparation time, making them a suitable solution for emergency situations.
Use of pre-filled syringes eliminates needle-stick and cut injuries when opening glass ampoules. (8)
Up to 86% of injectable drugs prepared in advance in the operating theater and intensive care are discarded at the end of the day. (9)
Considering all components used in the preparation steps of ampoules (syringe, drug, needle, pad, diluent…etc), pre-filled syringes could reduce your overall plastic use and the amount of waste you send to incinerators.